If Melting Point Obtained Allowed to Cool and Obtained Again Will It Be the Same

Ice cubes put in water will starting time to melt when they reach their melting point of 0 °C
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting indicate the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure level such as ane atmosphere or 100 kPa.
When considered every bit the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization signal. Because of the ability of substances to supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing betoken" of a substance is determined, in fact, the actual methodology is nearly always "the principle of observing the disappearance rather than the formation of ice, that is, the melting point."[1]
Examples [edit]

Melting points (in blueish) and humid points (in pinkish) of the first viii carboxylic acids (°C)
For most substances, melting and freezing points are approximately equal. For instance, the melting signal and freezing point of mercury is 234.32 kelvins (−38.83 °C; −37.89 °F).[ii] However, certain substances possess differing solid-liquid transition temperatures. For example, agar melts at 85 °C (185 °F; 358 M) and solidifies from 31 °C (88 °F; 304 Grand); such direction dependence is known as hysteresis. The melting bespeak of ice at 1 atmosphere of pressure is very close[three] to 0 °C (32 °F; 273 K); this is too known equally the ice indicate. In the presence of nucleating substances, the freezing point of water is not e'er the same as the melting signal. In the absence of nucleators water tin exist as a supercooled liquid down to −48.3 °C (−54.9 °F; 224.8 Grand) before freezing.
The metal with the highest melting point is tungsten, at three,414 °C (6,177 °F; 3,687 K);[iv] this property makes tungsten excellent for utilise as electric filaments in incandescent lamps. The often-cited carbon does not melt at ambient force per unit area but sublimes at well-nigh iii,700 °C (vi,700 °F; iv,000 K); a liquid stage only exists in a higher place pressures of 10 MPa (99 atm) and estimated 4,030–4,430 °C (seven,290–8,010 °F; iv,300–four,700 Chiliad) (see carbon phase diagram). Tantalum hafnium carbide (TaivHfC5) is a refractory compound with a very high melting point of iv,215 K (three,942 °C; vii,127 °F).[5] Quantum mechanical calculator simulations take predicted that the alloy HfN0.38C0.51 will have an even higher melting point (near 4400 K),[6] which would brand it the substance with the highest melting point at ambience pressure. This prediction was after confirmed by experiment.[7] At the other end of the scale, helium does non freeze at all at normal pressure fifty-fifty at temperatures arbitrarily shut to absolute zero; a pressure of more than twenty times normal atmospheric pressure is necessary.
| List of mutual chemicals | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemic[I] | Density ( k / cm3 ) | Melt (K) [8] | Eddy (One thousand) | |||||||||
| Water @STP | one | 273 | 373 | |||||||||
| Solder (Pb60Sn40) | 456 | |||||||||||
| Cocoa butter | 307.ii | - | ||||||||||
| Alkane series wax | 0.9 | 310 | 643 | |||||||||
| Hydrogen | 0.00008988 | fourteen.01 | 20.28 | |||||||||
| Helium | 0.0001785 | — [2] | 4.22 | |||||||||
| Glucinium | one.85 | 1560 | 2742 | |||||||||
| Carbon | ii.267 | — [III] [9] | 4000[3] [9] | |||||||||
| Nitrogen | 0.0012506 | 63.15 | 77.36 | |||||||||
| Oxygen | 0.001429 | 54.36 | xc.20 | |||||||||
| Sodium | 0.971 | 370.87 | 1156 | |||||||||
| Magnesium | one.738 | 923 | 1363 | |||||||||
| Aluminium | 2.698 | 933.47 | 2792 | |||||||||
| Sulfur | 2.067 | 388.36 | 717.87 | |||||||||
| Chlorine | 0.003214 | 171.6 | 239.11 | |||||||||
| Potassium | 0.862 | 336.53 | 1032 | |||||||||
| Titanium | 4.54 | 1941 | 3560 | |||||||||
| Fe | 7.874 | 1811 | 3134 | |||||||||
| Nickel | 8.912 | 1728 | 3186 | |||||||||
| Copper | 8.96 | 1357.77 | 2835 | |||||||||
| Zinc | 7.134 | 692.88 | 1180 | |||||||||
| Gallium | 5.907 | 302.9146 | 2673 | |||||||||
| Silverish | 10.501 | 1234.93 | 2435 | |||||||||
| Cadmium | eight.69 | 594.22 | 1040 | |||||||||
| Indium | 7.31 | 429.75 | 2345 | |||||||||
| Iodine | 4.93 | 386.85 | 457.iv | |||||||||
| Tantalum | 16.654 | 3290 | 5731 | |||||||||
| Tungsten | 19.25 | 3695 | 5828 | |||||||||
| Platinum | 21.46 | 2041.4 | 4098 | |||||||||
| Aureate | 19.282 | 1337.33 | 3129 | |||||||||
| Mercury | xiii.5336 | 234.43 | 629.88 | |||||||||
| Pb | eleven.342 | 600.61 | 2022 | |||||||||
| Bismuth | 9.807 | 544.7 | 1837 | |||||||||
| Notes
| ||||||||||||
Melting point measurements [edit]

Kofler bench with samples for calibration
Many laboratory techniques exist for the conclusion of melting points. A Kofler bench is a metal strip with a temperature gradient (range from room temperature to 300 °C). Any substance can be placed on a section of the strip, revealing its thermal behaviour at the temperature at that point. Differential scanning calorimetry gives information on melting bespeak together with its enthalpy of fusion.

Automatic digital melting indicate meter
A basic melting point apparatus for the analysis of crystalline solids consists of an oil bath with a transparent window (most basic design: a Thiele tube) and a uncomplicated magnifier. Several grains of a solid are placed in a thin glass tube and partially immersed in the oil bath. The oil bath is heated (and stirred) and with the assist of the magnifier (and external light source) melting of the individual crystals at a certain temperature can be observed. A metal block might be used instead of an oil bathroom. Some modern instruments have automatic optical detection.
The measurement can likewise exist made continuously with an operating process. For instance, oil refineries measure the freeze point of diesel fuel "online", meaning that the sample is taken from the process and measured automatically. This allows for more than frequent measurements as the sample does non accept to be manually collected and taken to a remote laboratory.
Techniques for refractory materials [edit]
For refractory materials (e.k. platinum, tungsten, tantalum, some carbides and nitrides, etc.) the extremely high melting point (typically considered to be to a higher place, say, 1800 °C) may be determined past heating the material in a black body furnace and measuring the black-body temperature with an optical pyrometer. For the highest melting materials, this may require extrapolation by several hundred degrees. The spectral radiance from an incandescent body is known to exist a function of its temperature. An optical pyrometer matches the radiance of a body nether study to the radiance of a source that has been previously calibrated equally a function of temperature. In this way, the measurement of the absolute magnitude of the intensity of radiation is unnecessary. However, known temperatures must be used to determine the calibration of the pyrometer. For temperatures above the scale range of the source, an extrapolation technique must be employed. This extrapolation is accomplished past using Planck'due south law of radiation. The constants in this equation are not known with sufficient accurateness, causing errors in the extrapolation to get larger at college temperatures. However, standard techniques take been developed to perform this extrapolation.
Consider the case of using gold as the source (mp = 1063 °C). In this technique, the current through the filament of the pyrometer is adjusted until the light intensity of the filament matches that of a black-body at the melting point of gold. This establishes the chief scale temperature and tin be expressed in terms of electric current through the pyrometer lamp. With the same current setting, the pyrometer is sighted on some other black-body at a college temperature. An absorbing medium of known transmission is inserted between the pyrometer and this blackness-torso. The temperature of the black-body is and then adjusted until a match exists between its intensity and that of the pyrometer filament. The truthful college temperature of the black-body is then determined from Planck's Police force. The arresting medium is then removed and the current through the filament is adapted to match the filament intensity to that of the black-body. This establishes a 2d calibration signal for the pyrometer. This step is repeated to carry the calibration to college temperatures. Now, temperatures and their corresponding pyrometer filament currents are known and a curve of temperature versus current tin be drawn. This curve can so be extrapolated to very loftier temperatures.
In determining melting points of a refractory substance by this method, it is necessary to either have black body atmospheric condition or to know the emissivity of the material being measured. The containment of the high melting material in the liquid state may introduce experimental difficulties. Melting temperatures of some refractory metals have thus been measured past observing the radiation from a black body cavity in solid metal specimens that were much longer than they were wide. To course such a cavity, a hole is drilled perpendicular to the long centrality at the heart of a rod of the material. These rods are then heated by passing a very big electric current through them, and the radiation emitted from the hole is observed with an optical pyrometer. The point of melting is indicated by the darkening of the pigsty when the liquid phase appears, destroying the black body conditions. Today, containerless light amplification by stimulated emission of radiation heating techniques, combined with fast pyrometers and spectro-pyrometers, are employed to allow for precise command of the time for which the sample is kept at extreme temperatures. Such experiments of sub-second duration address several of the challenges associated with more traditional melting indicate measurements made at very high temperatures, such equally sample vaporization and reaction with the container.
Thermodynamics [edit]
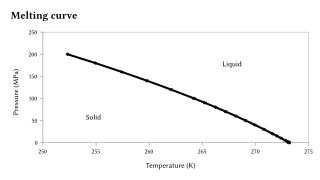
Pressure dependence of h2o melting betoken.
For a solid to melt, heat is required to raise its temperature to the melting point. However, farther rut needs to be supplied for the melting to take identify: this is chosen the heat of fusion, and is an case of latent heat.
From a thermodynamics point of view, at the melting betoken the alter in Gibbs free energy (ΔG) of the material is zero, simply the enthalpy (H) and the entropy (S) of the material are increasing (ΔH, ΔS > 0). Melting phenomenon happens when the Gibbs free energy of the liquid becomes lower than the solid for that textile. At various pressures this happens at a specific temperature. It tin likewise be shown that:
Here T, ΔS and ΔH are respectively the temperature at the melting point, change of entropy of melting and the change of enthalpy of melting.
The melting indicate is sensitive to extremely big changes in force per unit area, but more often than not this sensitivity is orders of magnitude less than that for the boiling point, because the solid-liquid transition represents merely a small change in volume.[10] [xi] If, every bit observed in most cases, a substance is more dense in the solid than in the liquid country, the melting point will increase with increases in pressure. Otherwise the opposite behavior occurs. Notably, this is the instance of water, every bit illustrated graphically to the right, but also of Si, Ge, Ga, Bi. With extremely big changes in pressure, substantial changes to the melting point are observed. For instance, the melting point of silicon at ambient pressure (0.1 MPa) is 1415 °C, but at pressures in backlog of 10 GPa it decreases to grand °C.[12]
Melting points are frequently used to characterize organic and inorganic compounds and to define their purity. The melting point of a pure substance is always higher and has a smaller range than the melting point of an impure substance or, more generally, of mixtures. The higher the quantity of other components, the lower the melting betoken and the broader will be the melting point range, often referred to as the "pasty range". The temperature at which melting begins for a mixture is known as the "solidus" while the temperature where melting is complete is called the "liquidus". Eutectics are special types of mixtures that carry like unmarried phases. They melt sharply at a constant temperature to grade a liquid of the same composition. Alternatively, on cooling a liquid with the eutectic limerick will solidify every bit uniformly dispersed, small (fine-grained) mixed crystals with the same limerick.
In contrast to crystalline solids, glasses exercise not possess a melting betoken; on heating they undergo a polish drinking glass transition into a viscous liquid. Upon farther heating, they gradually soften, which tin can be characterized by sure softening points.
Freezing-betoken depression [edit]
The freezing indicate of a solvent is depressed when another chemical compound is added, meaning that a solution has a lower freezing point than a pure solvent. This phenomenon is used in technical applications to avert freezing, for example by adding table salt or ethylene glycol to h2o.
Carnelley's rule [edit]
In organic chemistry, Carnelley's dominion, established in 1882 past Thomas Carnelley, states that high molecular symmetry is associated with high melting bespeak.[13] Carnelley based his rule on examination of xv,000 chemical compounds. For case, for three structural isomers with molecular formula C5H12 the melting point increases in the serial isopentane −160 °C (113 Thousand) n-pentane −129.8 °C (143 K) and neopentane −16.iv °C (256.8 K).[14] Also in xylenes and as well dichlorobenzenes the melting point increases in the gild meta, ortho and and then para. Pyridine has a lower symmetry than benzene hence its lower melting point simply the melting point once again increases with diazine and triazines. Many muzzle-like compounds similar adamantane and cubane with high symmetry accept relatively high melting points.
A high melting point results from a high oestrus of fusion, a low entropy of fusion, or a combination of both. In highly symmetrical molecules the crystal phase is densely packed with many efficient intermolecular interactions resulting in a higher enthalpy change on melting.

Like many high symmetry compounds, tetrakis(trimethylsilyl)silane has a very high melting betoken (m.p.) of 319-321 °C. It tends to sublime, then the chiliad.p. determination requires that the sample exist sealed in a tube.[15]
Predicting the melting bespeak of substances (Lindemann's benchmark) [edit]
An attempt to predict the majority melting point of crystalline materials was first fabricated in 1910 by Frederick Lindemann.[sixteen] The idea backside the theory was the observation that the boilerplate aamplitude of thermal vibrations increases with increasing temperature. Melting initiates when the aamplitude of vibration becomes large enough for adjacent atoms to partly occupy the same space. The Lindemann criterion states that melting is expected when the vibration root mean square amplitude exceeds a threshold value.
Assuming that all atoms in a crystal vibrate with the aforementioned frequency ν, the average thermal energy can be estimated using the equipartition theorem as[17]
where m is the atomic mass, ν is the frequency, u is the boilerplate vibration amplitude, g B is the Boltzmann constant, and T is the accented temperature. If the threshold value of utwo is ctwoa2 where c is the Lindemann constant and a is the atomic spacing, and then the melting point is estimated every bit
Several other expressions for the estimated melting temperature tin can exist obtained depending on the estimate of the average thermal energy. Another ordinarily used expression for the Lindemann benchmark is[xviii]
From the expression for the Debye frequency for ν, we have
where θ D is the Debye temperature and h is the Planck constant. Values of c range from 0.15 to 0.3 for near materials.[19]
Melting betoken prediction [edit]
In Feb 2011, Alfa Aesar released over 10,000 melting points of compounds from their itemize as open up data. This dataset has been used to create a random wood model for melting point prediction which is now freely bachelor.[xx] Open melting point data are also available from Nature Precedings.[21] Loftier quality data mined from patents and also models[22] adult with these information were published by Tetko et al.[23]
Table [edit]
| Group → | 1 | 2 | iii | four | 5 | 6 | seven | viii | 9 | ten | 11 | 12 | xiii | fourteen | fifteen | 16 | 17 | 18 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ↓ Menstruation | | ||||||||||||||||||||||
| one | H2 xiii.99 Grand (−259.16 °C) | | He 0.95 K (−272.20 °C) | ||||||||||||||||||||
| 2 | Li 453.65 Thou (180.50 °C) | Be 1560 Grand (1287 °C) | | B 2349 K (2076 °C) | C | N2 63.23 K (−209.86 °C) | O2 54.36 K (−218.79 °C) | F2 53.48 K (−219.67 °C) | Ne 24.56 M (−248.59 °C) | ||||||||||||||
| iii | Na 370.944 Chiliad (97.794 °C) | Mg 923 Yard (650 °C) | | Al 933.47 K (660.32 °C) | Si 1687 K (1414 °C) | P 317.iii K (44.15 °C) | S 388.36 Thousand (115.21 °C) | Cl2 171.half-dozen Thousand (−101.five °C) | Ar 83.81 K (−189.34 °C) | ||||||||||||||
| 4 | M 336.7 Thousand (63.v °C) | Ca 1115 K (842 °C) | Sc 1814 Yard (1541 °C) | Ti 1941 Thousand (1668 °C) | Five 2183 K (1910 °C) | Cr 2180 Yard (1907 °C) | Mn 1519 K (1246 °C) | Atomic number 26 1811 One thousand (1538 °C) | Co 1768 Grand (1495 °C) | Ni 1728 Thousand (1455 °C) | Cu 1357.77 K (1084.62 °C) | Zn 692.68 Chiliad (419.53 °C) | Ga 302.9146 Thou (29.7646 °C) | Ge 1211.xl K (938.25 °C) | As | Se 494 Thousand (221 °C) | Br2 265.viii K (−7.2 °C) | Kr 115.78 K (−157.37 °C) | |||||
| 5 | Rb 312.45 K (39.30 °C) | Sr 1050 K (777 °C) | Y 1799 G (1526 °C) | Zr 2128 Chiliad (1855 °C) | Nb 2750 Thou (2477 °C) | Mo 2896 K (1623 °C) | Tc 2430 Chiliad (2157 °C) | Ru 2607 K (2334 °C) | Rh 2237 Thou (1964 °C) | Pd 1828.05 K (1554.9 °C) | Ag 1234.93 K (961.78 °C) | Cd 594.22 Chiliad (321.07 °C) | In 429.7485 K (156.5985 °C) | Sn 505.08 K (231.93 °C) | Sb 903.78 K (630.63 °C) | Te 722.66 Thousand (449.51 °C) | Itwo 386.85 K (113.seven °C) | Xe 161.40 Grand (−111.75 °C) | |||||
| vi | Cs 301.vii K (28.five °C) | Ba 1000 Grand (727 °C) | | Lu 1925 K (1652 °C) | Hf 2506 One thousand (2233 °C) | Ta 3290 G (3017 °C) | W 3695 K (3422 °C) | Re 3459 K (3186 °C) | Bone 3306 K (3033 °C) | Ir 2719 K (2446 °C) | Pt 2041.iv G (1768.3 °C) | Au 1337.33 K (1064.eighteen °C) | Hg 234.3210 K (−38.8290 °C) | Tl 577 K (304 °C) | Atomic number 82 600.61 K (327.46 °C) | Bi 544.7 K (271.5 °C) | Po 527 Thousand (254 °C) | At 575 K (302 °C) | Rn 202 K (−71 °C) | ||||
| vii | Fr 300 K (27 °C) | Ra 973 Thousand (700 °C) | | Lr 1900 Chiliad (1627 °C) | Rf 2400 K (2100 °C) | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn 283±11 Thousand (10±11 °C) | Nh 700 Chiliad (430 °C) | Fl 200 K (−73 °C) | Mc 670 K (400 °C) | Lv 637–780 G (364–507 °C) | Ts 623–823 G (350–550 °C) | Og 325±xv K (52±15 °C) | ||||
| | |||||||||||||||||||||||
| | La 1193 K (920 °C) | Ce 1068 K (795 °C)6 | Pr 1208 K (935 °C) | Nd 1297 One thousand (1024 °C) | Pm 1315 Yard (1042 °C) | Sm 1345 M (1072 °C) | European union 1099 G (826 °C) | Gd 1585 K (1312 °C) | Tb 1629 Thousand (1356 °C) | Dy 1680 Grand (1407 °C) | Ho 1734 K (1461 °C) | Er 1802 1000 (1529 °C) | Tm 1818 K (1545 °C) | Yb 1097 K (824 °C) | |||||||||
| | Air-conditioning 1500 M (1227 °C) | Th 2023 K (1750 °C) | Pa 1841 K (1568 °C) | U 1405.iii Grand (1132.two °C) | Np 912±iii K (639±iii °C) | Pu 912.5 K (639.4 °C) | Am 1449 M (1176 °C) | Cm 1613 K (1340 °C) | Bk 1259 K (986 °C) | Cf 1173 K (900 °C) | Es 1133 K (860 °C) | Fm 1800 K (1527 °C) | Md 1100 Thou (827 °C) | No 1100 Thou (827 °C) | |||||||||
| Legend | |||||||||||||||||||||||
| Values are in Kelvin K and Celsius °C, rounded | |||||||||||||||||||||||
| For the equivalent in Fahrenheit °F, see: Melting points of the elements (information page) | |||||||||||||||||||||||
| Some values are predictions | |||||||||||||||||||||||
| Primordial From disuse Synthetic Edge shows natural occurrence of the element
| |||||||||||||||||||||||
See also [edit]
- Congruent melting
- Hagedorn temperature
- Highest melting indicate
- Liquidus
- Listing of elements by melting point
- Melting points of the elements (data page)
- Phase diagram
- Phases of matter
- Simon–Glatzel equation
- Slip melting point
- Solidus temperature
- Triple signal
- Zone melting
References [edit]
Citations [edit]
- ^ Ramsay, J. A. (1 May 1949). "A New Method of Freezing-Bespeak Determination for Small Quantities". Periodical of Experimental Biology. 26 (i): 57–64. doi:10.1242/jeb.26.1.57. PMID 15406812.
- ^ Haynes, p. 4.122.
- ^ The melting point of purified water has been measured every bit 0.002519 ± 0.000002 °C, meet Feistel, R. & Wagner, W. (2006). "A New Equation of Land for H2O Water ice Ih". J. Phys. Chem. Ref. Data. 35 (2): 1021–1047. Bibcode:2006JPCRD..35.1021F. doi:x.1063/1.2183324.
- ^ Haynes, p. 4.123.
- ^ Agte, C. & Alterthum, H. (1930). "Researches on Systems with Carbides at High Melting Point and Contributions to the Problem of Carbon Fusion". Z. Tech. Phys. 11: 182–191.
- ^ Hong, Q.-J.; van de Walle, A. (2015). "Prediction of the material with highest known melting betoken from ab initio molecular dynamics calculations". Phys. Rev. B. 92 (2): 020104(R). Bibcode:2015PhRvB..92b0104H. doi:10.1103/PhysRevB.92.020104.
- ^ Buinevich, V.S.; Nepapushev, A.A.; Moskovskikh, D.O.; Trusov, G.Five.; Kuskov, One thousand.Five.; Vadchenko, S.One thousand.; Rogachev, A.S.; Mukasyan, A.S. (March 2020). "Fabrication of ultra-high-temperature nonstoichiometric hafnium carbonitride via combustion synthesis and spark plasma sintering". Ceramics International. 46 (10): 16068–16073. doi:10.1016/j.ceramint.2020.03.158. S2CID 216437833.
- ^ Holman, Due south. Westward.; Lawrence, R. R.; Barr, L. (i January 1895). "Melting Points of Aluminum, Argent, Gilt, Copper, and Platinum". Proceedings of the American Academy of Arts and Sciences. 31: 218–233. doi:10.2307/20020628. JSTOR 20020628.
- ^ a b "Carbon". rsc.org.
- ^ The exact relationship is expressed in the Clausius–Clapeyron relation.
- ^ "J10 Heat: Change of aggregate state of substances through alter of heat content: Change of aggregate state of substances and the equation of Clapeyron-Clausius". Retrieved 19 February 2008.
- ^ Tonkov, E. Yu. and Ponyatovsky, E. G. (2005) Stage Transformations of Elements Under High Pressure, CRC Printing, Boca Raton, p. 98 ISBN 0-8493-3367-nine
- ^ Brownish, R. J. C. & R. F. C. (2000). "Melting Point and Molecular Symmetry". Journal of Chemical Education. 77 (half dozen): 724. Bibcode:2000JChEd..77..724B. doi:10.1021/ed077p724.
- ^ Haynes, pp. 6.153–155.
- ^ Gilman, H.; Smith, C. 50. (1967). "Tetrakis(trimethylsilyl)silane". Journal of Organometallic Chemistry. eight (2): 245–253. doi:10.1016/S0022-328X(00)91037-iv.
- ^ Lindemann FA (1910). "The calculation of molecular vibration frequencies". Phys. Z. 11: 609–612.
- ^ Sorkin, S., (2003), Signal defects, lattice structure, and melting, Thesis, Technion, Israel.
- ^ Philip Hofmann (2008). Solid country physics: an introduction. Wiley-VCH. p. 67. ISBN978-three-527-40861-0 . Retrieved 13 March 2011.
- ^ Nelson, D. R., (2002), Defects and geometry in condensed affair physics, Cambridge University Press, ISBN 0-521-00400-4
- ^ Predict melting indicate from SMILES. Qsardb.org. Retrieved on thirteen September 2013.
- ^ Bradley, Jean-Claude; Lang, Andrew; Williams, Antony; Curtin, Evan (eleven August 2011). "ONS Open up Melting Point Collection". Nature Precedings. doi:10.1038/npre.2011.6229.i.
- ^ OCHEM melting point models. ochem.eu. Retrieved on xviii June 2016.
- ^ Tetko, Igor 5; 1000. Lowe, Daniel; Williams, Antony J (2016). "The development of models to predict melting and pyrolysis point data associated with several hundred thousand compounds mined from PATENTS". Journal of Cheminformatics. 8: 2. doi:10.1186/s13321-016-0113-y. PMC4724158. PMID 26807157.
Sources [edit]
- Works cited
- Haynes, William G., ed. (2011). CRC Handbook of Chemical science and Physics (92nd ed.). CRC Press. ISBN978-1439855119.
External links [edit]
- Melting and boiling point tables vol. 1 by Thomas Carnelley (Harrison, London, 1885–1887)
- Melting and boiling signal tables vol. 2 by Thomas Carnelley (Harrison, London, 1885–1887)
- Patent mined data Over 250,000 freely downloadable melting point data. Likewise downloadable at figshare
Source: https://en.wikipedia.org/wiki/Melting_point





0 Response to "If Melting Point Obtained Allowed to Cool and Obtained Again Will It Be the Same"
Post a Comment